When put on the spot can you tell ‘how many milligrams of Lignocaine (lidocaine) there are in 5mls of a 1 percent solution?‘
Well, don’t worry if you are not sure, panic is most people’s initial thought. In healthcare the classic confusion exists with “%” solutions and “1:1000” dilutions.

In this post we would like to talk about drug dilutions for injectable medications. There is a particular focus on adrenaline (epinephrine). We set out a helpful introduction for average people like us to the supposedly simple yet confusing concepts.
KEY POINTS
- It is easy to confuse when rushing or in an emergency.
- It is critical to know Drug Dilution by heart for drugs such as Adrenaline (Epinephrine).
The Boring Bit
just 2 minutes (promise) of back to school chemistry:
- If you recall back to school, in chemistry class ‘weights’ rather than ‘volumes’ are often used in reference to chemical substances.
- Using weight of a chemical or drug can be confusing when we are dealing with medications that are diluted because diluted drugs have different concentrations
- In this post we make a Basic Assumption for the properties of fluids using water at 4 degrees celsius as an example (shown in the blue picture below)
What does this mean?
It means that 100mls of Water has a weight of 100g (at 4 Degrees Celsius**).
From this we can say that a 100% solution would be 1 gram of drug in 1ml of volume
Key Applications for Healthcare:
- There is 1 gram in 1 ml
- Therefore, as far as medications are concerned a 1 in 1 (1 gram per 1ml) Solution is the same as a 100% Solution. These Solutions have 100g of drug in 100mls of Fluid
- 1% is a common drug dilution. A 1% (the same as 1:100)
- This solution would have 1g per 100mls:
- There are 1000mg in 1 gram
- So this is also 1000mg per 100ml
- = 10
00mg per 100ml- = 10mg per 1ml
** Why 4 degrees? – Water is at its ‘most dense’ at 4 Degrees Celsius. Upon freezing, the density of water decreases by about 9% (If this was not the case the world would be a much different place where Ice would sink in your drink and to the bottom of the ocean). From our point of view the difference between 4 degrees and room temperature where drugs are stored is minimal
We will talk more below about this below in terms of two important examples:
(1) Lignocaine – ‘1%’ (can also be known as 1:100 dilution)
(2) Adrenaline (epinephrine) – ‘0.1%’ (also commonly known as 1:1000 dilution)
If you have time have these drug vials to hand to look at as you read the rest of this post.
Concentration
(Percentages and Ratios)
Medications in solution can be used for injection (e.g. IM, IV, SC, IO, IT) and administered by the various other routes.
Medications in solution have various different concentrations and potencies (e.g. IV adrenaline (epinephrine) is very potent and therefore a vial is relatively ‘dilute’). ‘Potency’ refers to the amount of drug required to produce an effect. Some drugs produce a powerful effect at minute doses (0.01 grams of IV adrenaline) and others need a large dose to have a therapeutic effect for our patient (5 grams of Magnesium)
As a result, because some drugs are very dilute and others are relatively concentrated, a confusing descriptive naming system has arisen .
Diluted Drugs could be written in the following ways by a prescriber:
- Please give 10mls of 1 in 10000 Adrenaline (epinephrine) IV to the patient (e.g. in a Cardiac Arrest)
- Please give 10mls of 1 percent subcutaneous Lignocaine (e.g. local anaesthetic for wound closure)
- Please give 20mmol of Magnesium Sulphate IV over 20 mins (e.g. Severe Asthma or Eclampsia in pregnancy)
This is already problematic and we have only listed 3 common emergency department drugs:

The Ratio Dilutions Explained
What does 1 percent, 1:1000 and 1:10000 refer to?
- 1 percent, 1:1000 and 1:10000 refers to the concentration of dilute drugs like adrenaline (epinephrine)
- 1 percent is the same as a 1:100 Solution
- 1:1000 is the same as 0.1% Solution
- 1:10000 is the same a 0.01% Solution
- Because 0.01% is a ‘mouthful’ dilute solutions such as adrenaline often are described as a ratio rather than a percentage. Sadly, having different systems has added to confusion rather than made it easier for us.
- Pick up your vial of Adrenaline/Epinephrine now (or look at the picture above)…
- The essential information is printed on the vial:
- There is a 1ml vial and it contains 1mg of adrenaline
-
- In other words there is 1mg/ml of Adrenaline in the vial shown above
- We commonly use this for IM injections in anaphylaxis
- The essential information is printed on the vial:
How does this work?
- 1:1000 means there is 1gram in 1000mls
- This is the same as 1g per 1 litre (or 1000mg per 1000mls)
- Taking this down to actual therapeutic doses that are used in real life clinical practice we ‘divide by 1000’ – so there will be 1mg per 1ml
- There is 1mg per 1ml in a standard 1:1000 vial of Adrenaline as shown above
Why are there two concentrations of Adrenaline anyway?
- 1:1000
- This is useful where a low volume is required for IM or SC injections
- If you were having an anaphylactic reaction to the delicious shellfish you just ate I am sure you would prefer the IM injection as 0.5ml (0.5.mg) rather than 5mls of a more dilute solution…
- 1:1000 is also easy to use for “nebulising” adrenaline in cases of upper airway obstruction
- 1:10000
- This is useful for giving as an ‘intravenous push’ dose in the cardiac arrest patient
- It is easy to use for making up an infusion of Adrenaline quickly and easily
- A vial of 1:10000 Adrenaline is shown below
- Sometimes 1:10000 Adrenaline comes in a “mini-jet” for rapid administration

Another Example
- In the vial above there is 10ml of 1:10000 adrenaline
- How many ‘mg’ of Adrenaline is there in this vial?
- Write down your answer now…
Suggested Answer
- 1:10000 means there is 1g in 10000ml
- In other words there is 1000mg in 10000ml
- Take off the zeros and you are left with 1mg in 10mls
- Alternatively, simply remember that 1:1000 Adrenaline is 1mg/1ml and divide by ten as 1:10000 is ten times more dilute than the 1:1000 Adrenaline…
Where does the “%” thing come in to all this?
- Many injectable medications are prepared and given as a ‘percentage’ (we have discussed adrenaline above which is a notable exception due to its high potency)
- When you are using any medication the vial label will give information in terms of the number of milligrams and the concentration
- However, we still think it’s important to understand what a “1 percent” solution actually means

- Lignocaine 1% is commonly used for topical local anaesthesia
- A 1% solution literally means there is 1gram per 100mls
- In ‘old adrenaline money’ this is the same as a 1:100 solution dilution…
- So, if there is 1g per 100mls there is also 1000mg per 100mls
- If we divide this down there will be 10mg in 1ml
- The vial pictured above has 5ml (so there is 50mg in this 5ml in this vial)
In real life clinical practice this is absolutely vital to know because there is are maximum safe does of Lignocaine… Lignocaine is a Sodium Channel Blocker (type Ib anti-arrhythmic). It is an effective anti-arrhythmic as well as being useful as a safe local anaesthetic. When it is injected into the skin for local anaesthesia there is always some systemic absorption which has the potential to cause toxicity. There are two main ways to reduce toxicity. Firstly, risk can be reduced by pre-mixing adrenaline with the lignocaine which causes vasoconstriction and therefore reduces absorption. Secondly, we always avoid inadvertent IV injection by always aspirating before injecting.
Lignocaine Dosing for Local Anaesthesia
- The safe dose for Lignocaine is 3 – 5mg/kg for ‘plain’ lignocaine
- The safe dose is 7mg/kg when adrenaline is added
- This means than in a 70kg health man we could safely use 3mg x 70kg = 210mg
- The vials of 1% lignocaine have 50mg in 5ml – so we could more than safely use up to 4 vials for our procedure in a healthy 70kg patient

Some Other Confusing Medications
There are several other commonly used Emergency Department medications what are worth briefly reviewing.
These include Calcium, Magnesium and Sodium Bicarbonate.
Magnesium and Sodium Bicarbonate are usually prepared as solutions in ‘mmmol/L’ concentrations whereas Calcium is primarily prescribed as a ‘percentage’ solution in a number of mls. For Example medics often order 10 mmol of Magnesium or 10mls of 10% Calcium Gluconate. Now that you have read this post hopefully the numbers will be easier to work out.
Magnesium as an Example of what we have learned
Magnesium is commonly Prescribed in mmmol (UK and Australia) or in grams (USA)
Prescribed as Magnesium Sulphate – 49.3% solution (493mg/ml)
Now you know what this means!
SO why 49.3%? Lets work through some maths:
In a 5ml vial of 49.3% you have 10mmol of Magnesium Sulphate (This is about 2.47g of Magnesium)
Working:
49.3% is the same as – 49.3 grams in 100mls of water
This is 49,300mg in 100mls
Take away the extra Zeros:
This is the same as 493mg in 1ml
SO, How much Magnesium is in a 5ml vial?
493 x 5 = 2465mg (which is about 2.47grams)
So this is how we get 2.465 grams in 5mls
Indications for Magnesium in the Emergency Department may include:
- significant hypomagnesaemia
- atrial fibrillation
- torsades de pointes
- pre-eclampsia
- eclampsia
- asthma
- (some say it works for everything except male pattern baldness and hypermagnesaemia)
Here is our Local Protocol – Click Here
Here is an overview of ED magnesium use – Click Here
Calcium
Calcium is generally prepared as either Calcium Gluconate or Calcium Chloride
Both Calcium preparations come as a 10% solution
In 10% Calcium Gluconate there will be 100g per 1000ml (100mg per 1ml)
Usually the dose prescribed is 10ml of 10% Calcium Gluconate
There is 1g of Calcium Gluconate in 10ml = 2.2 mmol in 10 mL solution
There is a nice discussion of Calcium and Hyperkalaemia at EMCRIT which we think is worth reviewing
Indications for Calcium in the Emergency Department include hyperkalaemia, hypocalcaemia, massive transfusion and magnesium toxicity.
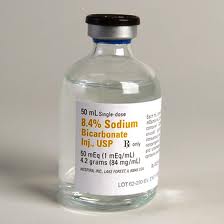
Sodium Bicarbonate
Various Concentrations are available including 1.26% and 8.4%
8.4% Sodium Bicarbonate is 84mg/ml
Each mL of solution contains 84.0 mg of sodium bicarbonate which gives 23.0 mg (or 1 mmol or 1 mEq) of sodium and 61.0 mg (or 1 mmol or 1 mEq) of bicarbonate. This is how they came up with 8.4%.
Indications for Sodium Bicarbonate in the Emergency Department include sodium channel blocking ‘drug toxicity’ and life threatening hyperkalaemia. The drug can also be used selectively (and controversially) in severe metabolic acidosis.
Note that this medication is hypertonic (2000mOsm/L) with a high sodium and alkalinising load so you’ll need to hyperventilate the patient to get rid of CO2 and keep an eye on the Sodium level.






Great blog Andy.
Great keep up the good work
Clear cut explanation… excellent
Good explanation
Thanks
Great Stuff
Great blog dude
Thank you very much for this wonderful information that I was looking for….
Very helpful…simple yet explicit…you don’t need anything more to understand drug dilution and concentration….thanks
I was in total confusion before reading this article itz very helpful thank you so much for the valuable knowledge in simple manner.
Nice blog – makes it easier to understnad
Awesome explanation – thank you
on behalf of my fellow math idiots everywhere, I thank and salute you. A godsend of an article.
Thanks, very kind
I can’t thank you enough!! Pulled all the “helpful tips ” from others together and showed the reason behind many short cuts!!
Thanks alot